This issue of VEEN was originally published as a .PDF in April 2024, and posted online. That issue is now archived, but accessible on the VEEN Archive webpage.

VEEN is curated by WSU Viticulture Extension. For questions on articles, or to request to submit an article in future issues, reach out to Michelle Moyer.
A Note from the Editor
While the 2024 spring is starting slightly ahead of last year, it has not been without its challenges. A wet early winter saw precipitation fall as rain, resulting in a low overall snow pack going into this season. A cold snap in January sent many of us scrambling to check buds – some regions and varieties made it through just fine, others were not so lucky. After a period of above-average temperatures, mid-April saw some light to killing frosts that nipped the shoots of our early-to-push varieties. Ultimately, this means we might be seeing lighter crops, and denser canopies (all of those latent buds pushing!) this season provided water is not overly-limited.
Resilience, though, is the name-of-the-game in Washington viticulture, and tough years provide us opportunities for reflection. Perhaps this means some deeper dives into variety and rootstock selection when considering a vineyard replant, or an adjustment to practices as you consider applying for the Sustainable WA certification. It is hard to hold down someone who chooses to move forward.
As you plan your pest management and nutrient programs for this year, consider pruning or canopy adjustments, and map out timing for cultural practices, squeeze in some time to enjoy this issue of VEEN. From nematodes to the Smart Vineyard, from Japanese Beetle to HiRes Vineayrd Nutrition, this issue has nuggets of good information and resource reminders!
Michelle M. Moyer
Viticulture Extension Specialist
Professor of Viticulture
WSU Prosser IAREC
Table of Contents
Digging into Meloidogyne hapla Sampling In Vineyards
Authors: Bernadette Gagnier and Michelle Moyer, WSU Prosser
Northern root-knot nematode (Meloidogyne hapla) is a soil-borne pest of Washington State wine grape (Vitis vinifera) vineyards. This nematode feeds on grapevine roots, which results in the formation of a gall that can restrict water and nutrient uptake (Fig. 1). In newly planted vineyards, the small root system can be quickly overtaken by feeding, leading to slow vine establishment and in severe cases young vine collapse. In established vines that have more robust root systems, long-term M. hapla feeding and infestation can be seen as slow, general vine decline.
The lifecycle of M. hapla begins as an egg located within a gelatinous mass typically on the outside of a root. It hatches as a second-stage juvenile (J2), having completed a molt in the egg. As a J2, it moves through soil seeking fine roots of a desirable plant host. Once a host root is identified, the J2 migrates into the root and establishes a feeding site near the vascular tissue of the root. Feeding from M. hapla causes the infected root cell to swell creating visible galls (Fig. 1). At this point the J2 becomes sedentary and molts through two additional stages into its adult form. Most adult M. hapla are female. The adults, which are inside the roots, lay eggs on the exterior of the root, which hatch and continue the cycle.
Meloidogyne hapla is most detrimental as a J2; this is the infective stage that can travel plant-to-plant. Given it’s also the stage that is present in the soil, it is what is sampled for diagnostics. Washington State has a model for predicting when M. hapla J2 are present in the soil [1]. This can inform when the best time of the season is to collect soil samples for M. hapla identification. The model is driven by soil temperature (at 8 inch depth), and uses a degree day calculation that is common in most crops. For M. hapla, the base temperature for this model is 0°C.

The model begins starts on March 1; our research has shown this is when J2 are at their peak population. By early summer, the J2 population density is at its lowest, as most have invaded plant roots and are developing into adults to lay eggs. Starting around July, these eggs begin to hatch, and J2 soil-borne populations start to increase. From a sampling perspective, this means there are two key time periods where sampling for M. hapla provides the most accurate picture of potential nematode pressure: the spring and the fall (Fig. 2).
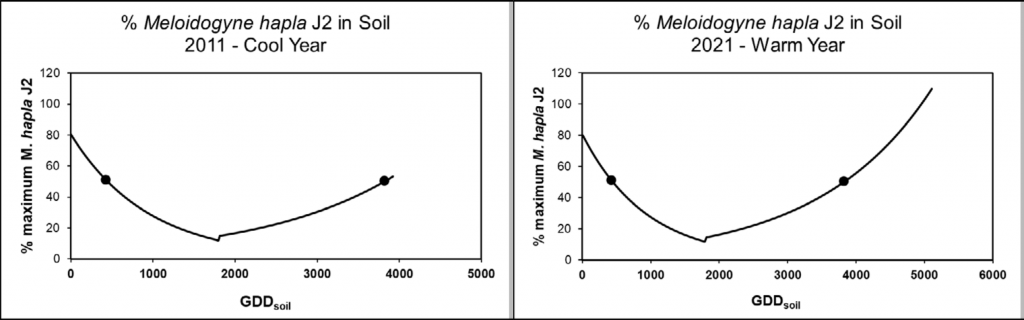
Currently, this model is not available on AgWeatherNet, but if you wish to calculate J2 populations, an Excel file is available to help you with that (email: michelle.moyer@wsu.edu). You can use AgWeatherNet daily average soil temperature for the model, or your own on-site soil temperature (from 8 inches soil depth). Because the model relies on daily GDDsoil, the calculated population dynamics of M. hapla will be site and time specific.
We recommend sampling when J2 populations are predicted to be at 50% or higher, as that improves your chances of detection. Other tips for sampling include:
- Sample under vines, near drip emitters. This is where fine feeder roots tend to grow, making it prime real-estate for M. hapla
- This nematode is most often in the top 24 inches of the soil [2]
- Dry soil can make sampling very difficult, if possible, irrigate a “full set” or at least 8 hours before sampling to ensure probes can reach adequate soil depth.
For more details, please refer to the Field Guide for Integrated Pest Management in Pacific Northwest Vineyards [3].
REFERENCES
- East et al. 2019a doi:10.1094/PDIS-07-18-1195-RE.
- East et al. 2019. doi:10.5344/catalyst.2019.19001
- Moyer & O’Neal. (eds). 2023. PNW Extension Publication #PNW644.
Cutting-Edge Research with HiRes Vineyard Nutrient Management
Authors: Nataliya Shcherbatyuk (WSU Prosser) and Patricia Skinkis (Oregon State University)
Welcome to HiRes Vineyard Nutrient Management! Here our cutting-edge research meets practical solutions for optimizing vine health and vineyard management.
We are team of scientists from seven institutions nationwide and we are dedicated to revolutionizing the way grape growers monitor and manage vineyard nutrition. Vineyard nutrient management is essential for maintaining vine health, productivity, and fruit quality targets. Our goal is to design field-based sensors and tools to measure relative grapevine nutrient status that may replace leaf and petiole sampling on the farm in the future. The intent is for these tools to provide growers with real-time vineyard nutrient status that can be used for precision management practices. If you’d like to dig deeper into our groundbreaking projects and the latest research findings, visit our website: https://highresvineyardnutrition.com.
Stay connect with us on social media, where we share updates on project activities and results:
- Instagram – https://www.instagram.com/hi_res_vineyardnutrition/
- LinkedIn – https://www.linkedin.com/company/hires-vineyard-nutrition/
- X – https://twitter.com/HiresVineyard
Have questions or comments? Don’t hesitate to reach out to our knowledgeable project members (https://highresvineyardnutrition.com/who-we-are/).
However that’s not all – you can also dive deeper into vineyard nutrition with our HiRes Vineyard Nutrition Podcast (https://extension.oregonstate.edu/podcast/hires-vineyard-nutrition-podcast)!
Dr. Patty Skinkis, Viticulture Extension Specialist and Professor at Oregon State University is our podcast host who delves into the technical and applied aspects of our research.
Subscribe to our podcast or access audio and written transcripts on the Oregon State University Extension website linked above.
This research is made possible by the National Grape Research Alliance (NGRA) and funded through the National Institute of Food and Agriculture’s Specialty Crop Research Initiative Coordinated Agricultural Projects (CAP) grant (Project Award Number: 2020-51181-32159).
Overview of Spotted Lanternfly (SLF)
Authors: Joshua M. Milnes and Cassie Cichorz, Washington State Department of Agriculture – Plant Protection Division

Status of Spotted Lanternfly in the United States
The spotted lanternfly (SLF), Lycorma delicatula (White) (Hemiptera: Fulgoridae) is native to most of East Asia and was first introduced into Barck County, Pennsylvania, U.S. in 2014 [4,7]. Since then, this planthopper has been reported in 17 U.S. states (established in 14 states) in just a very short time [8].
This planthopper is of major concern to growers in that it is a pest of many high-valued specialty crops including grapes, hops, tree fruit, plant nurseries, and timber industries [2,4]. Spotted lanternflies use their piercing/sucking mouthparts to feed on the nutrient rich phloem (sap) of plants. This is problematic as plants rely on phloem to transport vital nutrients to the rest of the plant.
In Washington, there have been no reports of SLF, but that can change quickly given the relative ease of unintentionally transporting this pest. At stake for Washington grape growers: over 57,000 acres of wine grapes. Add another 17,000 acres of juice grapes and Washington State becomes the second leading producer of all grapes in the U.S. As of 2022, overall grape production (about 412,000 tons) in Washington is up 40% from 2021 [3].
Spotted Lanternflies are Good Hitchhikers
What makes this invasive insect dangerous is its ability to spread. Spotted lanternfly likely hitchhiked to the U.S. on imported goods from Aisa. There are documented cases of people spreading this by moving infested materials [8]. The bug does not discriminate. It will lay its egg masses on almost any surface: shipping containers, vehicles, trailers, patio furniture, outdoor equipment, etc. SLF are weak flyers, but they more than make up for this by how well they walk / jump. There have been anecdotes of SLF moving an average of 12 miles per year. It is likely that SLF will enter Washington State via routes of entry from shipping, rail, tourism, or people moving here from the East Coast. Without proactive measures, the chances of an SLF introduction will increase every year. With the already well-established tree-of-heaven population and other closely related hosts, SLF will have no difficulty establishing in existing grape industry regions [9].
Spotted lanternfly has yet to be established on the West Coast. However, 11 dead SLFs have been detected on commercial airplanes and trucks coming from the East Coast to California [6]. Fortunately for the industry, they died in transit. California also recently intercepted an outdoor sculpture headed for California wine country which was found to be infested with numerous egg masses. This raises concern that there is a continued risk of their introduction to the West Coast from either the United States or East Asia and is very likely sooner than later. If SLF gains a foothold in the Pacific Northwest, it would likely be very difficult to control them given the insect’s pliability in both habitat and range.
Economic Impact
WSDA and USDA take SLF very seriously because of the economic impact they have on commodity crops. Many of these include agricultural specialty crops such as grapes, hops, tree fruit. In fact, a recent report by Penn State economists stated that SLF could potentially drain Pennsylvania’s economy of at least $324 million annually. In Washington State, the grape industry cannot afford this type of economic loss. According to Wakie, et al., 2020, the dry Eastern Washington region is optimal for the establishment of SLF. Millions of dollars of specialty crops would be at risk if SLF successfully hitchhikes to and throughout Washington State and costly quarantines would be established.
What has Been Done to Prevent the Tntroduction of SLF in Washington State?
On the East Coast, prevention tactics have been put into place to slow the spread of SLF, such as quarantines to restrict the movement of plant, wood, and stone products [4]. However, because the SLF is infamous for hitchhiking at all stages (particularly the cryptic egg masse stage), it has proven to be quite difficult to regulate the movement of “goods” potentially harboring them. Because of this, WSDA and USDA are monitoring SLF in Washington.
The good news is that the Washington State Department of Agriculture (WSDA) has been proactive in working with other state agencies to make things difficult for SLF to establish in our communities. WSDA has been teaming up with local county noxious weed boards to clean out the tree-of-heaven (TOH; one of the favorite hosts of SLF) in high-risk locations. Railroads, ports, parks are areas that would be optimal for the bug to establish.
WSDA is committed to protecting our agricultural commodities and has been surveying for SLF for over five years via a visual survey (funds provided by USDA APHIS). Grape surveys have come up with negative reports for SLF detections, which is great news for the industry!
WSDA, in collaboration with Washington State Invasive Species and other regulatory departments have teamed up to develop an action plan that contains steps to counter this pest from a regulatory level.
We also do extensive outreach to educate the public and industry about this pest, how to identify it and where to report it if someone thinks they have seen SLF. We’ve also worked with the Washington Invasive Species Council to promote public reporting of tree-of-heaven, to help document TOH populations. It is all about how “we” can help protect our community.
How Can You Help?
- Education: Learn how to identify SLF (Link: Take the time to see what they look like)
- Look out for SLF: Inspect outdoor items such as firewood, vehicles, and outdoor furniture for egg masses.
- Don’t spread it: If you visit other states with SLF, be sure to check all equipment and gear before leaving. Scrape off any egg masses you see and don’t bring them into Washington State!
- Photograph and report the pest: When you see something, take a photo and report it to the Washington Invasive Species Council or the WSDA Pest Program.
- When detected: Report, report, report! Collect specimens or egg masses so that WSDA can confirm whether or not the specimen is SLF. If unable to collect specimens or egg masses, squish nymphs and adults and scrape egg masses into soapy water.
WSDA is committed to working with the greater Pacific Northwest community and both state and federal programs to prevent the introduction of SLF in Washington, rapidly respond if it is detected here, and to protect our grape and hop industry from this invasive pest. If you see something suspicious, report it to WSDA through email at: pest@agr.wa.gov or calling 1-800-443-6684.
References
- Barringer, L. and C. Ciafré. 2020. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 49:999-1011.
- Francese, J. A., Cooperband, M. F., Murman, K. M., Cannon, S. L., Booth, E. G., Devine, S. M., & Wallace, M. S. 2020. Developing traps for the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Environ. Entomol. 49(2), 269-276.
- NASS. USDA. GOV. 2023. Washington annual statistical bulletin. United States Department of Agriculture National Agricultural Statistics Service Northwest Regional Field Office, Olympia, WA.
- Urban, J. M., and H. Leach. 2023 Biology and Management of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. 68:151-67.
- Pennsylvania Department of Agriculture. 2024. https://prdagriculture.pwpca.pa.gov/Plants_Land_Water/PlantIndustry/Entomology/spotted_lanternfly/quarantine/Pages/default.aspx. [Article Access: April 4, 2024].
- Rieger T. 2019. Dead Spotted Lanternflies Found at California Airports. Wine Business. https://www.winebusiness.com/news/article/221037 [Article Access: April 02, 2024].
- Spichiger, S-E. 2014. New Report, Berks County PA. Pennsylvania Department of Agriculture, Entomology. 22 Sept., 3pp.
- United State Department of Agriculture – Animal and Plant Health Inspection Service. 2024. Spotted Lanternfly. https://www.aphis.usda.gov/plant-pests-diseases/slf. [Article Access: April 01, 2024].
- Wakie, T. T., Neven, L. G., Yee, W. L., and Lu, Z. 2020. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 113: 306-314.
Japanese Beetle Quarantine Boundaries – Regulations Expanded
Authors: Cassie Cichorz, Washington State Department of Agriculture – Plant Protection Division
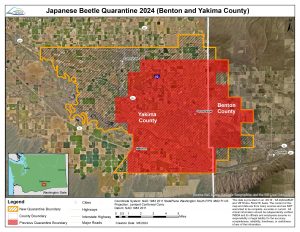
An emergency rule to expand the boundaries of the Japanese beetle quarantine in the Grandview and Sunnyside areas was filed on March 7 by the Washington State Department of Agriculture (WSDA).
After reviewing trapping data from the 2023 season, officials determined additional measures are needed to help curb the spread of the infestation while teams work to rid the area of this invasive beetle.
The rule expands the quarantine to encompass new areas where beetles have been caught, primarily in Sunnyside and west of Grandview. The map pictured shows the previously adopted quarantine area and the new boundaries established by the emergency rule.
Soil samples were added as regulated articles and the rule specifies conditions governing the movement of soil samples from quarantined areas to non-quarantined areas. The emergency rule also requires businesses located in the quarantine area selling regulated articles under WAC 16-470-710(4) or (7) to post signage developed by WSDA clearly stating that regulated articles purchased cannot be transported outside of the quarantined area.
The emergency rule amendment clarifies that under WAC 16-470-710(7), “cut flowers for decorative purposes” includes those flowers that are exposed to open-air environments during their harvest, transportation, or trade. Lastly, the amendment adds a condition for the transport of cut flowers grown in the quarantined area to areas outside the quarantined area.
Emergency rules are in effect immediately and last for 120 days. Officials will also begin the process of making the emergency rule permanent.
A Japanese beetle quarantine handout is available. Click the links below to download the resource.
- Japanese Beetle Quarantine Fact Sheet For Growers [PDF 1 MB
- Japanese Beetle Quarantine Fact Sheet For Growers in Spanish [PDF 1 MB]
Background
For the past three years, WSDA has taken extensive measures to reduce the spread of the beetle with the goal of eradicating it. These measures include treating area properties with a pesticide, trapping, and establishing a quarantine. These efforts reduced Japanese beetle catches in 2023 compared to the years prior; however, detections were found over a wider area including as far away as Pasco.
Identifying Japanese Beetle
Adult Japanese beetles are metallic green and brown and have little tufts of white hair on their sides. They emerge – usually from lawns or in other soil – in the spring and feed throughout the summer. From fall to spring the grubs (larvae) overwinter in the soil and slowly develop into mature adults ready to emerge again in the spring.
How Can I Help?
Community members can help by trapping, reporting, and killing the beetles on their properties. Residents and businesses must also follow the quarantine to prevent spreading the beetles by not moving items known to transport beetles outside of the quarantine area.
To limit the need to move yard debris and other plant material outside the quarantine area, WSDA has established a drop-off site available during the adult flight season, May to October. Businesses and residents can take all accepted items to the Japanese Beetle Response Yard Debris Drop-Off at 875 Bridgeview Rd., Grandview, WA 98930. There is no charge for disposal.
Those moving out of the quarantine area will not be able to take any of the regulated items with them.
Feedback Requested
We want to learn how these rule changes could impact you, your family, your agricultural business, or your community. Additionally, we want to hear your ideas on how we can reduce the impact of the quarantine on you or your community.
Please contact Jill Wisehart to share your thoughts on how we can mitigate further impacts on your community, at jwisehart@agr.wa.gov or 360-878-0298.
Smart Vineyard Concepts to Reality in Washington State
Authors: Jake Schrader and Lav Khot, WSU Prosser
This article provides a brief introduction to smart agriculture technology concepts, and some practical issues during their implementation in the Washington state vineyards for near-real-time crop monitoring and vineyard operations management.
Introduction
The USDA NIFA funded AgAID Institute, led by WSU, is aiming to develop artificial intelligence (AI)-powered tools to support on-farm decision making, and labor management in modern vineyards and orchards on the west coast. With the WSU’s Center of Precision and Automated Agricultural Systems (CPAAS), AgWeatherNet (AWN) and Viticulture & Enology (V&E) as key contributing units, a demo smart vineyard has been set up to demonstrate existing weather, soil and crop monitoring and data-driven vineyard operations management use-cases (e.g., Automated Deficit Irrigation, Heat Stress). This article scope is to discuss the aspects of sensing technology implementation and educate the stakeholders on terminology that they may come across.
Sensing & Data Infrastructure
Actionable data is data that can lead to a management decision. Frost management decisions are made based on temperature data. Pest management decisions are made based on population data tied with localized weather. Irrigation management decisions are made based on soil moisture data, along with weather and crop physiology data (if available). Data can come from a variety of sources, with some being arguably less subjective and noisy than others. Manual data collection, such as disease severity rating, introduces the potential for human error, giving less confidence in a management decision. One could thus use permanently installed georeferenced sensors, connected to a data logger and wireless network for real-time data transfer to the remote cloud servers for data storage, manipulation and to make management decisions from anywhere. The sensors can be connected to a data logger via a communication protocol (e.g., Recommended Standard 232 [RS232], Serial Digital Interface at 1200 baud rate [SDI-12]). For sensors generating time-series data, SDI-12 is one of the most integrable protocol that enables ease of developing a scalable data infrastructure ecosystem.
Typically, sensors generate changes in analog electrical signal based on changes in physical properties around them. The raw data generated by a sensor can be in the form of either voltage (mV), current (A), or occasionally time (s) between signals. For example, soil volumetric water sensors measure the time for an electrical signal to travel between two probes installed in the ground. The speed of the signal can then be correlated to the volumetric water content using a calibration equation. Calibration equations are used to relate these electrical signals to actionable data values. For SDI-12 sensors, these calibration equations can be applied within the sensor itself, enabling smoother integration into data management ecosystems. Otherwise, the analog signal must be converted to actionable data somewhere else along the data flow pipeline, realized through connectivity.
There are four primary wireless data communication techniques that can be used by agricultural technology providers (Fig. 1). The cellular 4G LTE/5G is the most prominent and reliable communication technique, provided good reception at the vineyard site. However, each of the device in the field will typically incur a subscription cost, similar to cell phones. To reduce costs, service providers may use a wireless network in the field such as Wi-Fi, Long-Range Wireless Area Networking (LoRaWAN) or BlueTooth to connect multiple devices to a single hub (i.e., gateway) that uses cellular connectivity for data transfer to a designated remote computer ( termed ‘cloud server’).
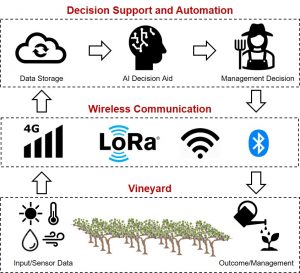
Often, digital data is not actionable without additional processing to contextualize it with the vineyard. For example, cumulative growing degree days (GDD) is a useful tool for tracking vine phenology through the growing season, but it is not directly measured. Calculations are required to transform temperature data into GDD data. This process can be automated through computing either at the edge (of vineyard) or in the (remote) cloud. Edge computing uses a microcomputer, also called single board computer, in the field to pre-process the data using low-level machine learning and can create application-dependent actuation triggers (e.g., actuate foggers to mitigate heat stress).
The primary advantage of edge computing is that it can reduce a large data source (i.e., an image) into a single value of actionable data, prior to sending to the cloud, reducing the cost of data transfer and cloud computing. However, edge computation requires rugged and reliable hardware to reduce the failure rate. If connectivity exists, cloud computing is often more reliable way to realize smart actuations. For either scenario with cyber connectivity, security of data and connected ag-tech in the vineyard needs due attention so growers don’t fall victim to ransomware or similar threats.
Commercially available localized sensing systems have the ability to help realize near-real-time decision support [1]. However, localized sensing systems require relevant historical data to contextualize the readings with respect to the decision at hand. Historical data is the primary tool growers have for decision-making. For example, the AgAID Institute is currently using historical weather data to create AI-powered grape cold hardiness models to allow greater confidence in management decisions. Such models can be tied to weather forecasting for better planning. Most agricultural weather and crop sensing service providers include some variation of crop/pest/disease modelling and weather forecasting. Many companies doing such forecasting are pulling data from an IBM forecasting source (the weather channel). This method of forecasting does not hold up well in the hills and valleys of the Columbia Basin. AWN station-specific forecasting is attempting to tackle these issues through installation of localized stations and refined spatial forecasting [2].
Smart Vineyard Implementation
In support of on-going automated deficit irrigation vineyard research, the vineyard block is instrumented with relevant sensors to understand the flow of water through the soil-plant-atmosphere continuum.
Most commonly, on commercial operations, we see automated irrigation controlled by soil sensors. Soil water content sensors measure the amount of water present per given volume of soil. Soil matrix potential sensors (also referred to as tensiometers) measure the negative pressure generated in response to plants removing water from the soil. Soil sensors are great for tracking temporal variability of water, especially for flat homogeneous sites. When soil moisture sensors are used for irrigation control, an experimental threshold based on historic experience is set to trigger irrigation events. This threshold is typically adjusted over time to encourage positive crop response to irrigation. For information on soil sensors and data interpretation, see [1, 3].
Atmospheric sensors measure weather parameters such as air temperature, wind speed, relative humidity and solar radiation which can all be used to estimate total evapotranspiration from the vine. This can be a good solution to estimate how much water a theoretical vine is using, but it doesn’t give an accurate estimate of how much total water is needed by the vineyard as a whole. There are losses that can’t be mitigated, runoff, deep percolation, consumption by weeds, so this approach requires some subjective adjustments after calculation.
Plant-based sensors have gained popularity as of late with multiple startup companies pushing single sensor-solutions for irrigation insights. Sap flow sensors are designed to track how much water is moving through the vine throughout the day. Dendrometers measure the nanoscale changes in trunk diameter and have been shown to correlate well to vine stress. Microtensiometers give a direct measurement of stem water potential indicating real-time plant water status and need for irrigation.
As digital agriculture is a relatively new field, there has been an explosion of startups and vendors offering sensing technologies and associated decision-support tools. While the sensing technology itself may provide new insights, vendors are not prioritizing integration of those sensors into scalable ecosystems. In the demo smart vineyard, we have sensors from five different companies needing vendor specific data acquisition and visualization subscriptions. These individual sensors transfer data to respective vendor managed remote cloud (server/computers) and industry-wide efforts are needed to make the sensors more integrable in generic ecosystem.
Our current efforts are thus focused on transitioning all sensors over to our LoRaWAN network to reduce subscription costs and to realize secured ecosystem of data transfer and analysis. With site-specific gateway directly connected into the WSU wireless network, we are able to reduce costs associated with multiple cellular subscriptions. However, by implementing our own system, we also have to develop our own data visualization dashboard which has proven to be challenging.
Learnings: Sensor Damage & Protection
It is not uncommon for tractors and machinery to cause damage to vineyard trellis wires, posts, and crops. Implement companies have innovated systems that avoid such damage. For example, use of retracting arms for under-vine tillage and optical sensors for pre-pruners to avoid posts. Machines are less developed for avoidance of sensing equipment (Fig. 2). Wires for soil moisture sensors fall prey to soil and floor management tools. Direct plant sensors can be broken by harvesters and canopy management machinery (e.g., pre-pruners, trimmers and leafers). Over-the-row machineries will destroy solar panels, antennae and other above canopy hardware.

Based on the past 2-year learning, below are some of the practical considerations for increasing longevity of field deployed sensors:
- Remove sensors from the field during mechanical operations. Possible for atmospheric, dendrometers, some non-destructive sap flow sensors. Less practical for soil moisture and destructive plant sensors.
- Flag vines with sensing systems. Requires more cognitive load for machine operator but may save time uninstalling and installing.
- Install protective barriers around sensors. Works for soil sensors and under vine management. Protective installations could influence accuracy of sensor if isolating too much from environment.
Acknowledgments
The ongoing projects are funded by USDA NIFA and the WA Grape and Wine Commission. The authors would also like to thank the Deficit Irrigation Research Team members: Shafik Kiraga, Dr. Troy Peters, Dr. Claudio Stockle, and Dr. Markus Keller. We would also like to thank AgAID Institute members Srikanth Gorthi, Dr. Paola Pesantez and Dr. Ananth Kalyanaraman. Finally, thanks to the WSU-CAHNRS IT department for guidance and assistance in setting up and maintaining digital infrastructure.
Additional Reading
- Peters, T. 2011. VEEN Spring 2011. Irrigation Sensors for Washington Vineyards.
- Cann, M., S. Hill and Khot, L., 2023. VEEN Spring 2023. AgWeatherNet Station-Specific 10-Day Weather Forecast Available!
- Jacoby, P. 2023. VEEN Fall 2023. Soil Water Sensors: Monitoring Water Use and Irrigation Scheduling.
Solutions in Science
Author: By Melissa Hansen, Research Program Director, Washington State Wine Commission
Washington State Wine Commission works to advance game-changing research through state, regional and national channels.
The Washington State Wine Commission, through its dedicated research funding, has created a robust research program that works to solve challenges of Washington wine grape growers and wineries. For each of the last seven years, the Washington State Wine Commission has awarded around $1M annually to fund important viticulture and enology research projects. In addition, we team up with fellow grape and wine organizations to support and initiate innovative research at local, regional, and national levels.
National Involvement
The Washington State Wine Commission serves on the board of directors of the National Grape Research Alliance (NGRA), a nonprofit membership organization representing the research needs of all sectors and regions of the American grape and wine industry. The National Grape Research Alliance spans wine, table grapes, juice, and raisins to connect industry, academic scientists, and federal and state agencies to initiate novel research projects and programs. The Washington Winegrowers Association was instrumental in founding the NGRA in 2005, and since then the group has secured $65 million for scientific solutions to grape and wine industry issues.
Several Washington State representatives serve on NGRA committees, including Washington State University’s Markus Keller and Michelle Moyer, Yun Zhang of Ste. Michelle Wine Estates, and Mark Amidon, representing National Grape Cooperative/Welch’s. In addition to serving on its board of directors, I chair the Integrated Production Systems Committee, known as the “Vineyard Tools” Committee.
A key role of the Alliance is to represent the research needs of the grape and wine industry to the U.S. Department of Agriculture’s Agricultural Research Service (ARS). Four NGRA committees biannually update research priorities and provide stakeholder input to ARS. Last November, the Alliance hosted a grape industry workshop for ARS in Beltsville, Maryland, where the agency is headquartered. The workshop shared research priorities and emerging challenges with federal scientists and leaders and launched new research projects and programs to address critical industry needs. Washington’s wine industry was represented by participation from the Washington State Wine Commission and the Washington Winegrowers Association.
Three research concepts emerged from the workshop, which are now being fleshed out into research proposals: 1) early detection of grape-related mealybug species for management of leafroll virus; 2) grapevine genetics for production efficiencies, focused on mechanization/automation/labor reduction and fruit quality traits for table grapes and raisins; and 3) climate change mapping and mitigation.
The Washington State Wine Commission and Washington State University collaborated on initial planning sessions of the early detection of mealybug species concept. As the concept takes shape of a research proposal, we will help move it forward for grant funding. This concept aligns with one of the Washington State Wine Commission’s strategic research pillars for the next five years of leading transformational research on leafroll virus.
Past NGRA-initiated Projects
In recent years, NGRA initiated the following research projects:
- Grape Varieties in the U.S.A.– is a web-based project of NGRA, led by Matthew Fidelibus, University of California, Davis, with funding from the American Vineyard Foundation.
- HiRes Vineyard Nutrition –led by Markus Keller, WSU, and initiated by NGRA, this project aims to develop new grapevine nutrient sensors for spatial mapping and variable rate nutrient management. The project received a $4.75 M grant from the U.S. Department of Agriculture’s Specialty Crop Research Initiative. The Washington wine industry jump-started the project and provided $90,000 to Dr. Keller’s research that was then leveraged into SCRI support.
- VitisGen3 – an NGRA-initiated project led by Matt Clark, University of Minnesota, focuses on genetics for disease resistance and is funded by a $10M grant from USDA’s Specialty Crop Research Initiative.
- Efficient Vineyard – a national effort to advance the use of precision agriculture for wine, juice and table grape production. Led by Terry Bates, Cornell University, the NGRA-initiated project was funded through USDA’s Specialty Crop Research Initiative and created the MyEV Tool, a free, web-based tool to help growers collect, process and use spatial data for their own vineyard management.
Building for the Future
The National Grape Research Alliance works to grow its own research fund to seed projects important to the industry.
Planning grants are provided to bring scientists together to propose multi-discipline and university projects and the organization recently created its own Fellowship program. The competitive program awards $30,000 per year for three years to an aspiring Ph.D. student in grape related science supporting an NGRA research priority. Through mentoring, field tours, networking, and providing support in the early stages of a promising scientist’s career, NGRA hopes to build a relationship that will last a lifetime.
The Washington State Wine Commission served as a mentor to the first NGRA Fellow, Abby Hammermeister, a graduate student at UC-Davis who is studying grapevine water stress indicators to better reflect holistic stress responses.
Regional Focus
Closer to home, the Washington State Wine Commission participates in the Northwest Center for Small Fruits Research, a partnership of Pacific Northwest researchers and stakeholders of grapes, wine and small fruits. The research-led consortium is funded by the USDA and was established to increase the sustainability and economic productivity of berry, grape, and wine industries of Washington, Oregon and Idaho.
The administrative headquarters of the center is located at Corvallis, Oregon, with major research programs at Washington State University, Oregon State University and University of Idaho.
Created in 1992, the Center provides a forum for Northwest small fruits producers, processors and wineries to address regional problems, set research funding priorities, and direct limited federal dollars to leverage state-funded programs.
In recent years, the Center has awarded about $1.5 M annually for berry, grapes and wine research and has funded viticulture and enology research conducted by Washington State University scientists.
The Washington State Wine Commission works at all levels, from local to regional to national, to stretch industry research dollars. Additionally, many WSU scientists stretch their research grant dollars by leveraging support from different funding agencies.
If you are interested in learning more about membership in the National Grape Research Alliance or how to connect with the Northwest Center for Small Fruits, please contact me at: mhansen@washingtonwine.org